NUCLEAR PHYSICS ( BINDING NUMERICALS )
- Calculate the binding energy per nucleon for:
- 208Pb (mass = 207.976652 u)
- 40Ca (mass = 39.962591 u)
- 56Fe (mass = 55.934939 u)
Use the following values:
- mass of proton = 1.007825 u
- mass of neutron = 1.008625 u
- 1 u = 931.5 MeV
Calculate the total binding energy (B.E.) of each nucleus. The binding energy is given by the equation: 𝐵.𝐸.=(𝑍⋅𝑚𝑝+𝑁⋅𝑚𝑛−𝑚nucl)×931.5 MeV/u
B.E.=(Z⋅mp+N⋅mn−mnucl)×931.5 MeV/u where:
- 𝑍 is the number of protons,
- N is the number of neutrons,
- mp is the mass of a proton (1.007825 u),
- mn is the mass of a neutron (1.008625 u),
- mnucl is the mass of the nucleus,
- 931.5 MeV/u is the conversion factor from atomic mass units to MeV.
Step 2: Divide by the number of nucleons to find the binding energy per nucleon.
For 208𝑃𝑏208Pb (Z = 82, A = 208):
- Number of neutrons 𝑁=𝐴−𝑍=208−82=126
- B.E.=(82×1.007825+126×1.008625−207.976652)×931.5 MeV
- 𝐵.𝐸.=(82.64145+127.08675−207.976652)×931.5 MeV
- B.E.=(1.751543)×931.5 MeV
- 𝐵.𝐸.=1631.85 MeV
- Binding energy per nucleon=2081631.85=7.84 MeV/nucleon
For 40𝐶𝑎40Ca (Z = 20, A = 40):
- Number of neutrons 𝑁=𝐴−𝑍=40−20=20
- B.E.=(20×1.007825+20×1.008625−39.962591)×931.5 MeV
- 𝐵.𝐸.=(20.1565+20.1725−39.962591)×931.5 MeV
- B.E.=(0.366409)×931.5 MeV
- 𝐵.𝐸.=341.41 MeV
- Binding energy per nucleon=40341.41=8.54 MeV/nucleon
For 56𝐹𝑒56Fe (Z = 26, A = 56):
- Number of neutrons 𝑁=𝐴−𝑍=56−26=30
- B.E.=(26×1.007825+30×1.008625−55.934939)×931.5 MeV
- 𝐵.𝐸.=(26.20345+30.25875−55.934939)×931.5 MeV
- B.E.=(0.527261)×931.5 MeV
- 𝐵.𝐸.=491.18 MeV
- Binding energy per nucleon=56491.18=8.77 MeV/nucleon
Thus, the binding energies per nucleon are:
- 208𝑃𝑏208Pb: 7.84 MeV/nucleon
- 40𝐶𝑎40Ca: 8.54 MeV/nucleon
- 56𝐹𝑒56Fe: 8.77 MeV/nucleon
- 2.Calculate the binding energy released in the following nuclear reactions:
- 235U → 141Ba + 92Kr + 3n
- 232Th → 136Xe + 94Sr + 2n
Use the following values:
- mass of 235U = 235.043929 u
- mass of 232Th = 232.038055 u
- mass of 141Ba = 140.914406 u
- mass of 92Kr = 91.926156 u
- mass of 136Xe = 135.907219 u
- mass of 94Sr = 93.915361 u
- mass of neutron = 1.008625 u
SOL
Reaction 1: 235U→141Ba+92Kr+3𝑛235U→141Ba+92Kr+3n
Reactant Mass:
- 235U=235.043929 u
Product Masses:
- 141Ba=140.914406 u
- 92Kr=91.926156 u
- 3 neutrons = 3×1.008625 u=3.025875 u
Total Mass of Products:
140.914406 u+91.926156 u+3.025875 u=235.866437 u
Mass Defect:
Δ𝑚=mass of reactants−mass of products
Δ𝑚=235.043929 u−235.866437 u
Δm=−0.822508u (negative value indicates a loss of mass)
Energy Released (Binding Energy):
𝐸=Δ𝑚×931.5 MeV/u
E=Δm×931.5MeV/u
E=−0.822508×931.5MeV
E=−766.065534MeV (Energy released as a positive value)
Reaction 2: 232Th→136Xe+94Sr+2𝑛232Th→136Xe+94Sr+2n
Reactant Mass:
- 232Th=232.038055
Product Masses:
- 136Xe=135.907219 u
- 94Sr=93.915361 u
- 2 neutrons = 2×1.008625 u=2.017250 u
Total Mass of Products:
135.907219 u+93.915361 u+2.017250 u=231.839830 u
Mass Defect: Δ𝑚=mass of reactants−mass of products
Δm=232.038055u−231.839830u
Δ𝑚=0.198225 u
Energy Released (Binding Energy): 𝐸=Δ𝑚×931.5 MeV/u
E=Δm×931.5MeV/u
E=0.198225×931.5MeV
E=184.6780425MeV (Energy released)
Summary:
- For the reaction 235U→141Ba+92Kr+3𝑛235U→141Ba+92Kr+3n, approximately 766 MeV of energy is released.
- Calculate the binding energy per nucleon for the following nuclei:
- 12C (mass = 12.000000 u)
- 20Ne (mass = 19.992435 u)
- 28Si (mass = 27.976927 u)
Use the following values:
- mass of proton = 1.007825 u
- mass of neutron = 1.008625 u
- 1 u = 931.5 MeV
sol :
B.E.=(Z⋅mp+N⋅mn−mnucl)×931.5 MeV/u where:
- 𝑍Z is the number of protons,
- 𝑁N is the number of neutrons (calculated as 𝐴−𝑍A−Z, with 𝐴A being the mass number),
- 𝑚𝑝mp is the mass of a proton (1.007825 u),
- 𝑚𝑛mn is the mass of a neutron (1.008625 u),
- 𝑚nuclmnucl is the mass of the nucleus,
- 931.5 MeV/u is the conversion factor from atomic mass units to MeV.
Step 2: Divide by the number of nucleons to find the binding energy per nucleon.
For 12𝐶12C (Z = 6, A = 12):
- Number of neutrons 𝑁=𝐴−𝑍=12−6=6N=A−Z=12−6=6
- 𝐵.𝐸.=(6⋅1.007825+6⋅1.008625−12.000000)×931.5 MeV
- 𝐵.𝐸.=(0.0987)×931.5 MeV
- 𝐵.𝐸.=91.93 MeV
- Binding energy per nucleon=91.9312=7.66 MeV/nucleon
For 20𝑁𝑒20Ne (Z = 10, A = 20):
- Number of neutrons 𝑁=𝐴−𝑍=20−10=10
- B.E.=(10⋅1.007825+10⋅1.008625−19.992435)×931.5 MeV
- B.E.=(0.172065)×931.5 MeV
- B.E.=160.36 MeV Binding energy per nucleon=160.3620=8.02 MeV/nucleon
For 28𝑆𝑖28Si (Z = 14, A = 28):
- Number of neutrons 𝑁=𝐴−𝑍=28−14=14
- B.E.=(14⋅1.007825+14⋅1.008625−27.976927)×931.5 MeV
- B.E.=(0.253373)×931.5 MeV
- 𝐵.𝐸.=236.06 MeV
- Binding energy per nucleon=28236.06=8.43 MeV/nucleon
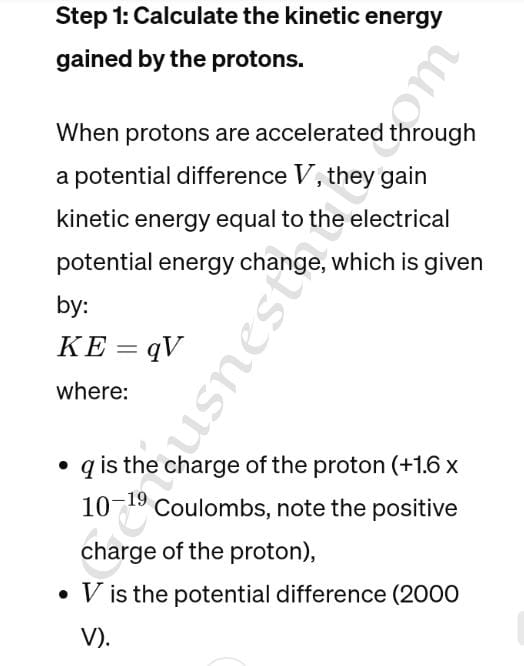
Q4.A beam of protons is accelerated from rest through a potential difference of 2000 V and then enters a uniform magnetic field which is perpendicular to the direction of the proton beam. If the flux density is 0.2 T, calculate the radius of the path which the beam describes. (Proton mass = 1.7 x 10^-27 kg, Electronic charge = -1.6 × 10^-19 C)
SOL;